Challenge
Embedded Linux in dental medicine
The electronic components and complex assemblies in W&H dental equipment must be mass-produced and assembled according to the strict standards of medical technology norms.

Intuitive operation by swiping, wiping, dragging - all of this is now also important in medical technology and is becoming more and more important. Disinfectable touch surfaces, high-quality workmanship and a secure software substructure are just a few of the numerous requirements that must be met and implemented here.
Medical technology is one of the key technologies of the 21st century. Electronics and embedded systems have become indispensable in medicine. Whether as wearable on the body, heart-lung machines in the hospital, diagnostic devices, laboratory equipment or treatment devices for the dentist. Electronics and software are omnipresent. Electronics and embedded systems are core components of today's medical technology, reduce costs and enable completely new areas of application.
Embedded Linux
W&H Dentalwerk Bürmoos GmbH
W&H Dentalwerk Bürmoos GmbH, headquartered in Bürmoos, develops, produces and markets precision instruments and devices for dental, surgical and dental technology applications. For this, the international company relies on Ginzinger and its embedded Linux solutions.
In 2014, the company, which employs over 1,200 people worldwide, celebrated the successful market launch of its first product with graphical user interface and embedded Linux as operating system, the surgical device "Piezomed".

Good reasons for the partnership
For the in-house development of the embedded system for the Piezomed, W&H lacked the technical know-how and also the human resources. For the company, it was clear from the beginning that they would outsource the development of the embedded Linux solutions they were looking for. "This realization was greater than the fear of maneuvering into a supply dependency," says Ing. Carina Schnaitl, developer at W&H.
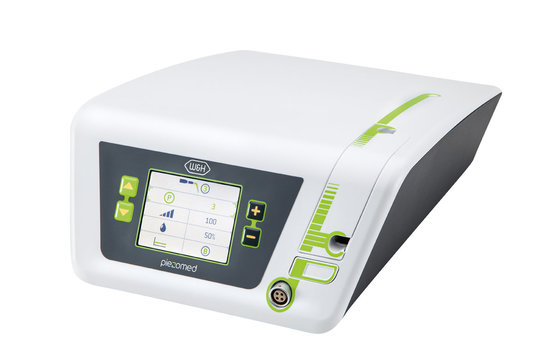
Innovative product in dental medicine
Piezomed from W&H
The Piezomed facilitates the work of oral surgeons and implantologists. Thanks to innovative ultrasonic technology, only bone substance is removed with high precision when implants are placed. The surrounding soft tissue remains unharmed. This means a noticeable relief for the dental surgeon as well as for the patient: Less pain, faster healing! The graphical user interface created by Ginzinger electronic systems, the appealing design and the embedded systems know-how guarantee quality and safety at all levels.
Ongoing further development
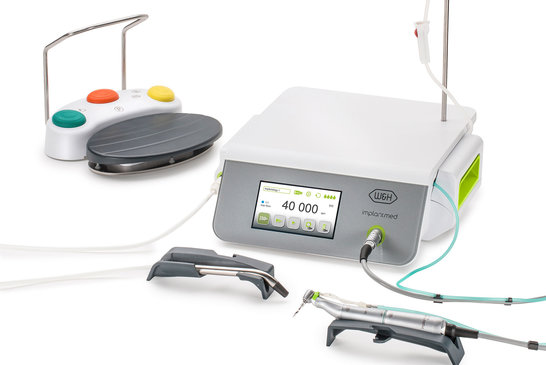
Implantmed from W&H
With the new Implantmed, another surgical unit with an intuitive operating concept, color touchscreen and glass surface followed in 2017. All electronic components and complex assemblies in W&H dental devices produced at Ginzinger must be mass-produced and assembled according to the strict standards of medical technology norms.
Together with the W&H experts, the developers are constantly working on the implementation of modern, graphical user interfaces. GELin, a Ginzinger Embedded Linux distribution proven over ten years, is used as the basis.
Problems are for solving
Converting challenges into solutions
The partnership between W&H and Ginzinger has been successful for several years now. Together, they strive to continuously develop the products further in order to remain competitive and leading on the market. On current problems in the production of flex circuit boards for W&H, Ginzinger was able to demonstrate its know-how and help its customer solve a problem:
When soldering a flex PCB for W&H, the test equipment manufacturing team tried to (find) a process to make the series producible faster. Background: Since it was not possible to solder contact pins with a size of 0.5mm on a flex PCB, an inductive soldering device was developed. The contact pins are positioned in a metal block, which is connected to the flexprint. The construction is then heated in the soldering device by induction, which solder the pins to the flexprint.

Important standards in medical technology
Medical Device Regulation MDR of the EU
Regulation (EU) 2017/745 on medical devices entered into force on May 25, 2017. It is also called Medical Device Regulation or European Medical Device Regulation. It applies in the member states of the European Union.
IVDR - In-vitro Diagnostic Device Regulation of the EU
The IVDR feels responsible for the entire in vitro diagnostics market in the EU: From development to market surveillance and application. It thus addresses manufacturers, importers, users as well as notified bodies and national authorities.
EN ISO 13485 - Management system for the design and manufacture of medical devic
ISO 13485 is an ISO standard that represents the requirements for a comprehensive quality management system for the design and manufacture of medical devices.
EN 60601 - set of standards on medical electrical equipment and systems
EN 60601 - set of standards on medical electrical equipment and systems